Cell News 04/2018
14
sister kinetochores attached correctly to the same spindle pole.
To our surprise, a large fraction of oocytes had one or more
rotated bivalent chromosomes, in which the sister kinetochores
of both pairs faced opposite spindle poles (Figure 4). Given that
the orientation of sister kinetochores in these rotated bivalents
is inverted, we called these bivalents ‘inverted bivalents’. We
also often observed half-inverted bivalents, in which only one
pair of the sister kinetochores was oriented towards opposite
spindle poles, while the other pair was correctly oriented. Im-
portantly, while around 12% of oocytes from women younger
than 30 showed at least one inverted bivalent, the incidence
of bivalent inversion increased to almost 40% in oocytes of
the advanced maternal age group. Altogether, these results
demonstrate that as women age and kinetochores progressively
separate, some oocyte’s chromosomes fail to maintain a correct
in-axis orientation. Segregation of such bivalents at anaphase
onset would likely result in profound chromosome segregation
errors, providing an additional explanation for why oocytes in
older women are particularly prone to aneuploidy
13
.
Developing new tools to study human-like aspects
of chromosome segregation
The work described above shed light to numerous interesting
features of the chromosome segregation machinery in human
oocytes that deserve further attention. Scarcity of human oo-
cytes available for research however continues to hinder such
investigation. While future studies performed directly in human
oocytes are likely to be pivotal for identifying new interest-
ing aspects of chromosome segregation machinery unique to
humans
12,13,15-17
, given the limited number of human oocytes, it
is unlikely that we will be able to fully investigate the mecha-
nistic basis of chromosome mis-segregation in this system in
the near future.
To circumvent this problem, I recently developed an experi-
mental setup that allows us to perform high-resolution studies
of chromosome behavior in a robust model system that more
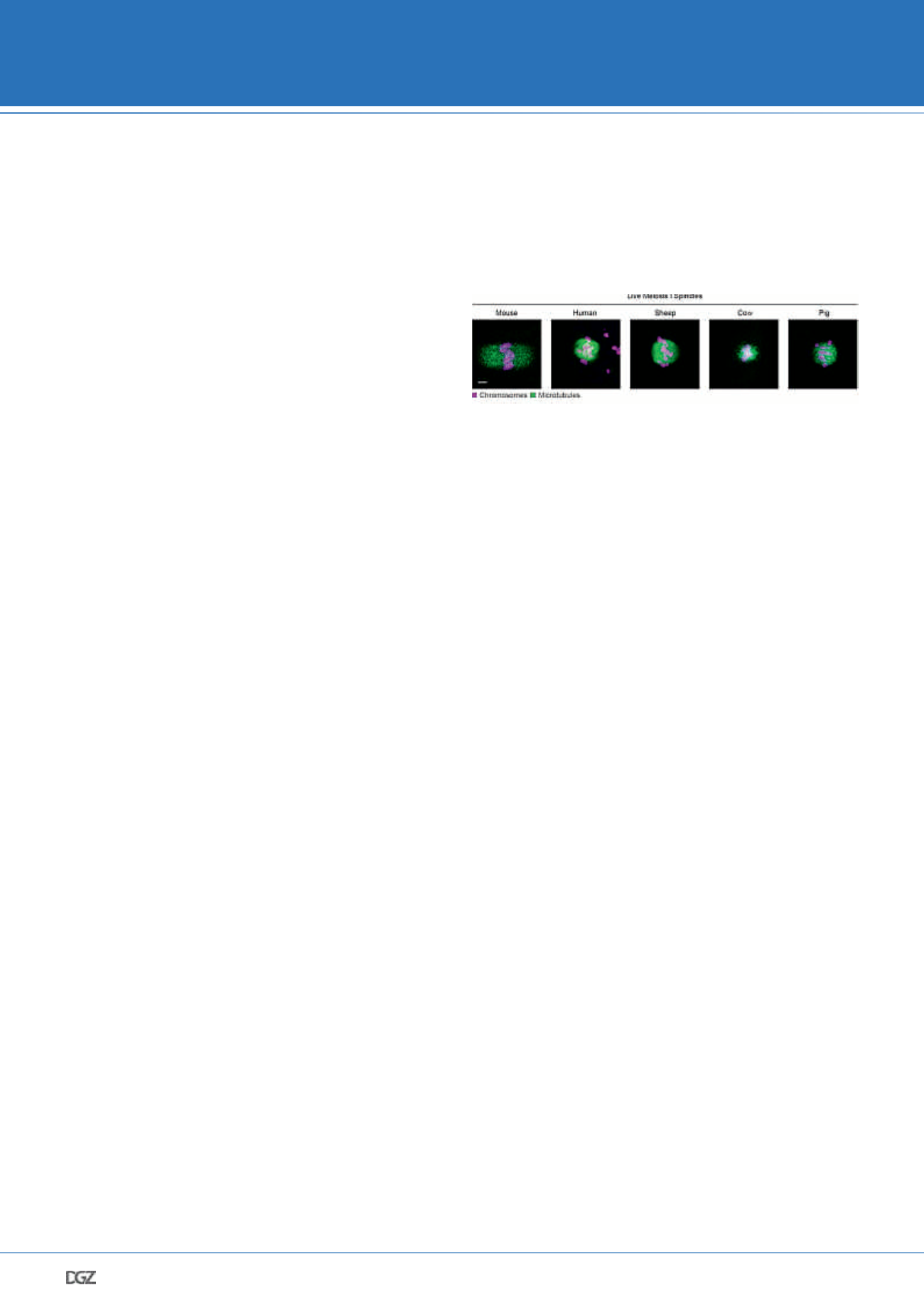
closely mimics human meiosis than mice do. Having investigat-
ed a plethora of non-rodent species, I scored for a number of
human-like features of meiotic progression (such as microtu-
bule dynamics and timing of key meiotic events) in live oocytes
from livestock mammals (Figure 5). This allowed us to study at
a mechanistic level some aspects of female meiosis that are
not conserved between human and mice, yet are represented
in other livestock mammals. Very importantly, this approach
allows one to study over 200 oocytes per experiment, which is
in stark contrast to the few human oocytes available to scien-
tific investigation at any given timepoint. Additionally, as the
livestock ovaries are obtained from local slaughterhouses and
would have otherwise been discarded, this approach also re-
sults in significantly fewer animals being sacrificed for research
purposes. Currently, we are exploiting this new system as a
human-like model to address fascinating mechanisms of chro-
mosome segregation that are otherwise very difficult to study
in human eggs. We hope that this system will significantly
accelerate our progress when answering pressing questions in
mammalian meiosis and human infertility.
Figure 5: Confocal microscopy images of live meiosis I spindles in
oocytes from five mammalian species
Representative images of live mammalian oocytes microinjected with fluo-
rescently labelled chromosome (H2B_mRFP or H2B_mScarlet; magenta) and
microtubule (MAP4_mEGFP; green) markers. Images shown are maximum
intensity projections of adjacent z-sections. Scale bar represents 5 µm.
Outlook
Why even young women frequently mis-segreate their chro-
mosomes during meiosis and how advancing maternal age
increases the likelihood of chromosome segregation errors have
long remained outstanding questions in developmental biolo-
gy
2
. Understanding the maternal-age effect is of an ever-rising
importance as an increasing number of women in Western
societies delay childbearing plans to their late thirties
3
. This
work elucidates previously unforeseen mechanisms of how
ageing affects chromosome architecture in human eggs and
opens new questions in the field of reproductive biology. It is
important to note that these observations were made using
surplus oocytes harvested from women undergoing Assisted
Reproductive Treatments, ART. While the extend to which ART
oocytes resemble those from naturally cycling women is yet
to be determined, the errors observed in women struggling to
conceive invariably shed invaluable insights into the aetiology
of human infertility. Implementation of modern microscopy
techniques, in conjunction with genetic studies and cross-spe-
cies approaches, is deemed to result in further breakthroughs
in the field and gives a grounded hope for new therapeutic
approaches to improve fertility treatments and help couples to
conceive.
Acknowledgements
I am very grateful to Dr Melina Schuh for her ongoing support
and putting so much trust in me from the very beginning of my
PhD. I thank all the past and present members of the Schuh Lab
at the MRC LMB in Cambridge and the MPI BPC in Göttingen
for creating a stimulating and truly enjoyable environment to
work in. The MB/PhD program at the University of Cambridge
for granting me an opportunity to intercalate a PhD in Cell
Biology into my Medical Degree. The Rosetrees Trust, Lister
Institute for Preventive Medicine, Medical Research Council,
European Research Council and the Max Planck Society for
funding this work. I would also like to acknowledge our IVF
NIKON YOUNG SCIENTIST AWARD 2018


