Cell News 04/2018
10
NIKON YOUNG SCIENTIST AWARD 2018
Female meiosis is exceptionally prone to errors
All mammalian life begins with the fertilization of an egg by
sperm
1
. When the egg and sperm fuse, a genetically unique
embryo is formed. Only high quality embryos can develop to
term and become a new, fully functional organism. Surpris-
ingly, even 20-70% of human embryos fail to meet this mark
2
.
Such poor quality embryos frequently contain an abnormal
number of chromosomes- they are aneuploid. Aneuploidy in
early embryos is in fact the leading cause of pregnancy loss
and several congenital disorders such as Down’s syndrome
2
.
The strikingly high predisposition of human embryos to
aneuploidy is indeed evident in clinical practice - even 1 in 7
couples in Western countries struggle to conceive
3
. To move
towards therapeutic interventions that will tackle the issue
of infertility affecting tens of millions of couples worldwide,
we first have to understand how embryos inherit an incorrect
number of chromosomes.
At fertilization, the genetic material of mum and dad unites
into a single cell. In fact, both gametes should contribute
exactly one copy of each chromosome to the newly formed
embryo
1
. However, while sperm cells are very robust, eggs fre-
quently fail to meet this target. This is because the process of
egg production from its precursor cell, the oocyte, is exception-
ally prone to errors
2
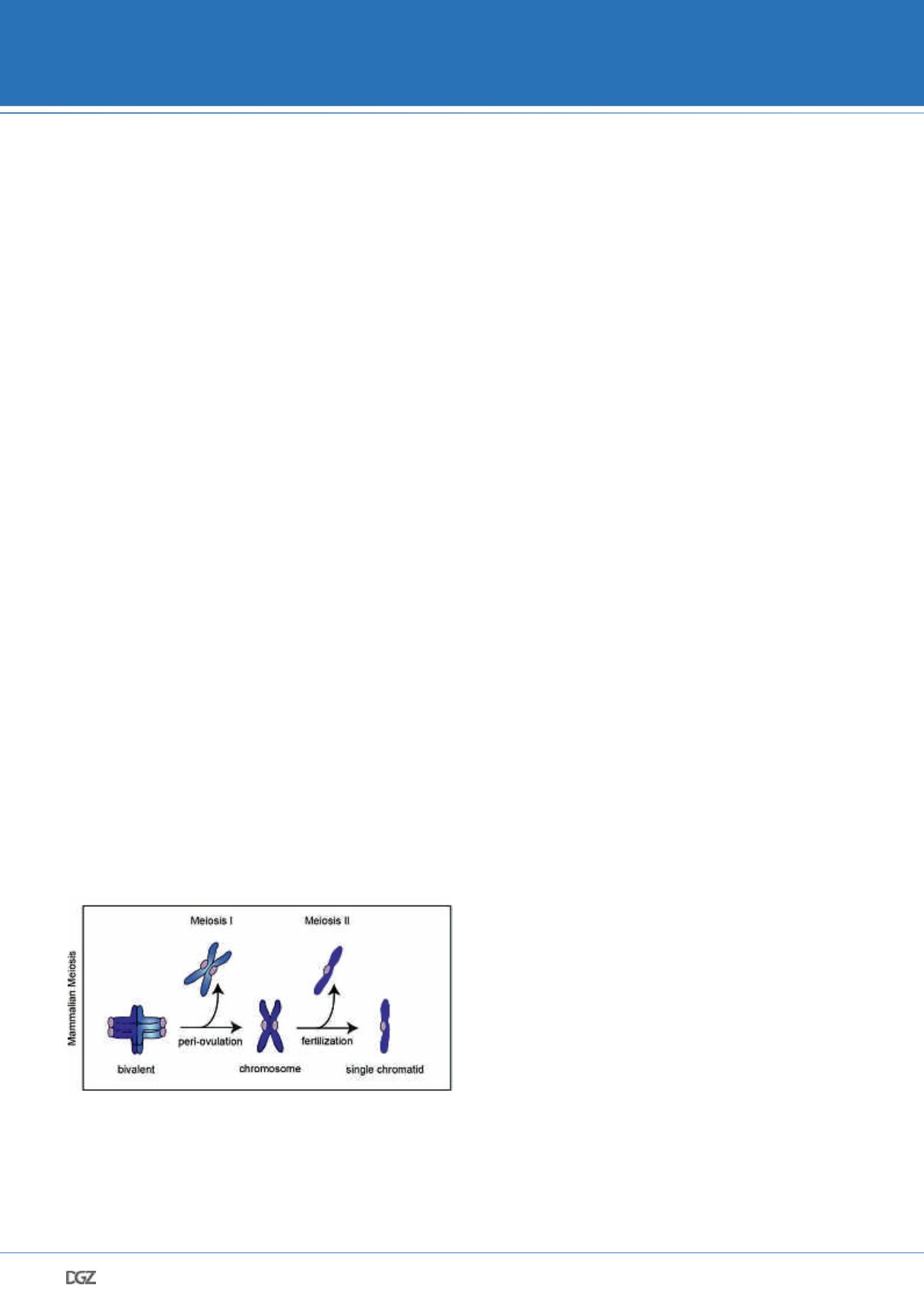
. In order to develop fully, the oocyte has
to segregate its chromosomes twice (Figure 1). During the first
meiotic division homologous chromosomes become separated,
whereas meiosis II partitions sister chromatids. It has been
demonstrated that both meiotic divisions in females are highly
erroneous. As a result, the egg frequently caries too many or
too few chromosomes, which severely impairs its potential to
contribute to a healthy pregnancy.
Figure 1: To complete development, the oocyte has to segregate its
chromosomes twice
Scheme illustrating the main events during oocyte maturation. The first chro-
mosome segregation event of the oocyte partitions the parental chromosomes,
so that only one chromosome of each pair remains in the egg. Subsequently,
individual chromosomes align on the second metaphase spindle. Fertilisation,
which predominantly occurs in the oviduct’s ampulla, triggers the second
chromosome segregation event, which partitions the two chromatids of each
chromosome. As a result, both the oocyte and the sperm provide a chromatid
of each pair to the future embryo. This cascade of events ensures the amount
of genetic material to be conserved between generations.
Oocyte quality declines profoundly with advancing
female age
Despite clear implications for human health, our understanding
of why meiosis in women is so unreliable is still limited. One
notable difference between the process of gamete production
in males and females is the timescale at which this process
occurs
1,2
. In males, mature sperm cells are produced continu-
ously on a demand basis once the individual reaches puberty.
In contrast, the prevailing view in the field is that the oocyte
pool in females is finite and non-replenishable from the time of
birth. Additionally, female eggs take over a decade to develop
fully
4,5
. This is because egg precursor cells are formed already
during the foetal life of a woman. These immature, prophase
arrested oocytes are then stored in the ovary until puberty.
Only thereafter, an oocyte resumes meiosis each menstrual
cycle and completes the first meiotic division. The egg then
arrests again as it awaits fertilization. Egg maturation is then
completed only upon sperm entry, which triggers the second
chromosome segregation event. Therefore, a human oocyte
takes even several decades to divide its chromosomes twice.
The significant protraction of meiosis in females creates a key
challenge. Not only is the oocyte’s chromosome segregation
machinery intrinsically prone to errors, but also the fidelity of
meiosis is subjected to a further decline as the woman gets
older. Indeed, genetic studies suggest that while already 20%
of eggs in women in their early twenties are chromosomally
abnormal, the incidence of faulty eggs increases even up to
50% as the woman reaches her forties
2,4,5
. The substantial
decline of egg’s quality with age is widely known as the ma-
ternal age-effect. Observations that stem from genetic studies
therefore pose two fundamental questions: firstly, why is the
chromosome segregation machinery frequently inefficient even
in young women. Secondly, what happens to the oocyte at a
molecular level as the women gets older that further impairs
meiotic fidelity. Importantly, understanding how oocytes
segregate their chromosomes is of great interest both from the
basic research point of view and is of an ever-rising clinical
relevance, as an increasing number of women in the Western
world delays childbearing plans until late thirties, a time when
oocyte quality is already suboptimal.
Studies in mice point to a crucial role for cohesin
loss in female ageing
It appears plausible that studying the chromosome segregation
machinery directly in human oocytes could reveal the mecha-
Agata Zielinska


