Cell News 04/2018
18
membrane domain and – with the exception of integrin
β
4 – a
short cytoplasmic tail, which governs the assembly of receptor
specific, intracellular signaling hubs. The resulting macromo-
lecular assemblies are known as focal adhesions (FAs; Fig. 2b)
and it is widely accepted that mechanotransduction of cell
adhesion is regulated in these complex structures (Geiger et al.
2009, Hoffman et al. 2011, Ringer et al. 2017). Yet, how forces
propagate in FAs was largely unclear.
To quantify molecular forces in FAs, we applied the tension
sensor technique to talin (Austen et al. 2015). Talin is ubiq-
uitously expressed and crucial for integrin-mediated cell
adhesion, because binding of its N-terminal head-domain to
β
-integrin subunits is required for the activation of virtually all
integrin receptor subtypes (Fig. 2a). The C-terminal rod-domain
of talin is made of thirteen helical bundles, which connect to
the actomyosin cytoskeleton by two actin binding sites as well
as eleven vinculin binding motifs (Roberts and Critchley 2009).
As mechanical tension across talin was able to induce vinculin
binding in vitro, talin was thought to function as a mecha-
nosensitive protein that translates mechanical information
(tension) into a biochemical output (vinculin recruitment to the
cell adhesion site) (del Rio et al. 2009).
We therefore inserted the TSM between the integrin-binding
head and the actin-binding rod domain of talin (Fig. 3a), and
we carefully tested whether talin is still functional after sensor
insertion. All our experiments indicated normal talin properties
suggesting that this approach could be used to read out molec-
ular forces at integrins’ cytoplasmic tail. By performing a whole
range of live cell FRET experiments, we could demonstrate that
talin experiences mechanical forces of about 7–10 pN during
cell adhesion indicated by specifically decreased FRET efficien-
cies as compared to control values (Fig. 3b, c). We then showed
that talin linkages are vinculin- and f-actin-dependent and
essential to sense the stiffness of the extracellular environ-
ment (Austen et al. 2015). In addition to these molecular force
measurements, we quantified how many talin molecules in a
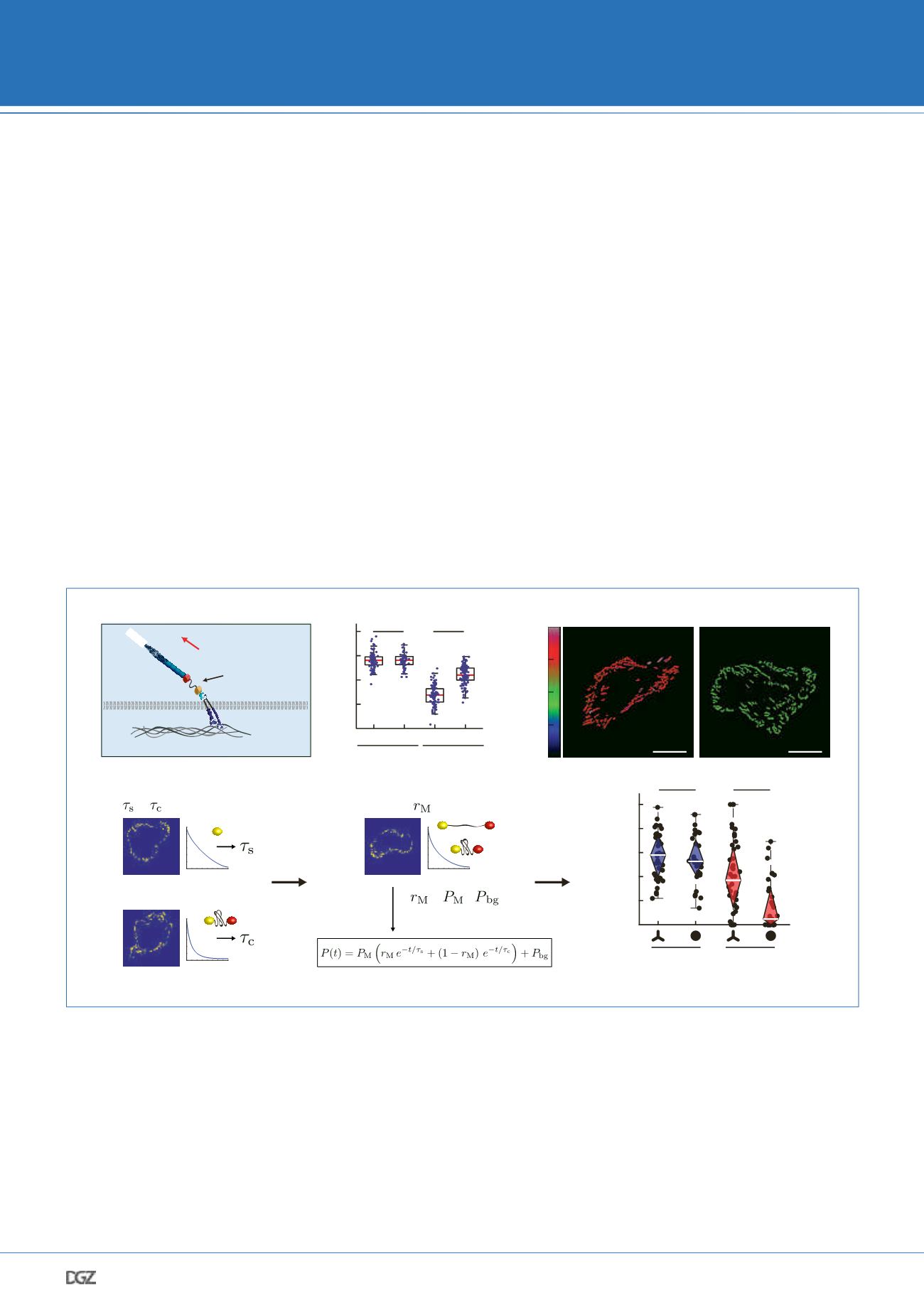
Figure 3: Analyzing integrin force transduction with talin tension sensors. a. Many cell types adhere to the extracellular matrix (ECM) in subcel-
lular structures called focal adhesions (FAs). The mechanical connection between integrins and the actin cytoskeleton is mediated by the integrin
activator talin. We developed a biosensor to quantify mechanical forces across this central FA protein. b. Representative data set of a live cell FLIM
experiment: While cells expressing the tension sensor control do not display differences when seeded on fibronectin (FN) or poly-Lysine (pL), talin
tension sensor expressing cells indicate reduced FRET efficiencies on FN-coated surfaces indicating molecular tension. c. We also use custom-writ-
ten software to determine FRET by ratiometric imaging. Shown are the expected differences between control and talin tension sensor expressing
cells indicating mechanical tension across talin-1. Scale bars indicate 20 μm. d. When using a TSM with digital force response characteristics (FL-
TSM), sensors can be assumed to be either in the open (no FRET) or closed (high FRET) state. Bi-exponential fitting of FL-TSM lifetime decays allows
the calculation of how many molecules in a given population are exposed to mechanical forces. e. About 60% of talin molecules experience tension
at N-terminal regions (aa 447) during cell adhesion on both triangular (high myosin activity) and round micropatterned substrates (low myosin ac-
tivity). Less molecules experience tension at the C-terminal insertion site (aa 1973) suggesting an intramolecular tension differential across talin-1.
FRET ratio [a.u]
0.00
FRET control
talin-1 sensor
0.50
0.35
0.17
0.70
FRET
FRET
FRET efficiency [%]
FN pL
30
40
20
10
FN pL
talin-1 sensor
FRET control
n.s.
***
intracellular
tension
f-actin
talin-1 sensor
extracellular matrix (ECM)
integrin
b
c
a
& determination
from bi-exponential fit
P
t
P
t
P
t
stretch ratio determination
,
,
Talin-YPet
Talin-Con
Talin-TSM
e
d
n.s.
***
N-terminal
region
C-terminal
region
engagement ration [%]
0
1.0
0.8
0.4
0.6
0.2
BINDER INNOVATION PRIZE 2018


