Cell News 04/2018
12
appeared as a single spot when subjected to similar imaging
conditions10.
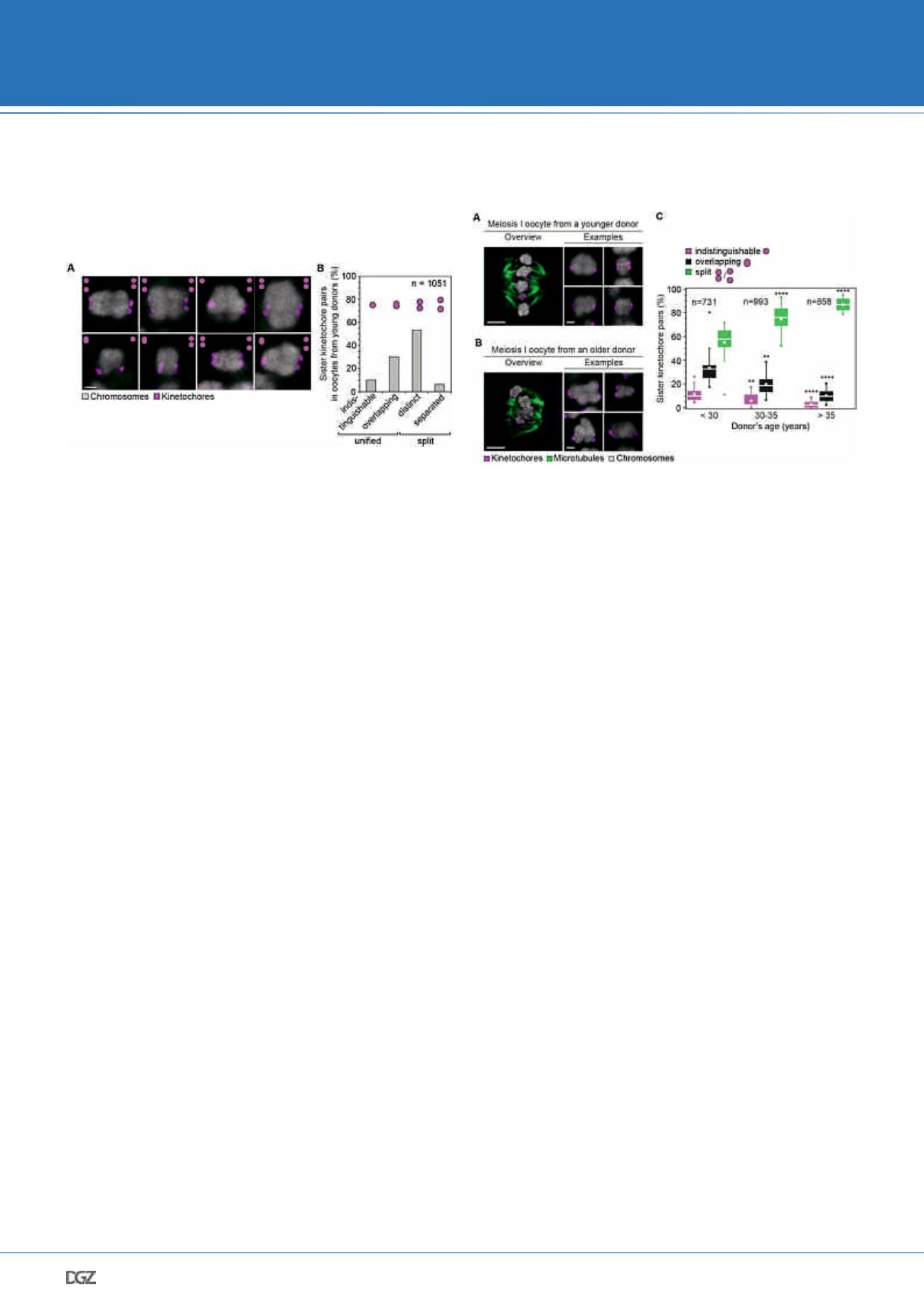
Figure 2: The majority of sister kinetochores during meiosis I are not
fully fused even in reproductively young women
(A) Representative images of meiosis I kinetochores and bivalents from five
young donors (
≤
30 years old). The images shown are maximum intensity
projections of all z-sections that contained the kinetochore signal. Scale bar
represents 1 µm.
(B) Frequency of kinetochore configurations as in (A) in meiosis I oocytes from
young donors (
≤
30 years old). 1,051 sister kinetochore pairs from 23 oocytes
were included in this analysis.
Importantly, the separation of kinetochores in human oocytes
had functional consequences- we were able to demonstrate
that separated sister kinetochores can interact independently
with spindle microtubules. As kinetochore-microtubule attach-
ments determine the directionality of chromosome movement
at anaphase onset, this indicated that the separated kineto-
chore geometry may be in a position to influence chromosome
segregation outcomes of human meiosis. Moreover, as kine-
tochore separation was evident already in oocytes from young
women, this observation could further provide an explana-
tion to why even women in their early twenties occasionally
mis-segregate their chromosomes.
Additionally, our analysis revealed that the degree of ki-
netochore individuality increases with maternal age, with
the average spacing between sister kinetochores becoming
progressively larger with every year of a women’s life (Figure
3). We further demonstrated that not only the two sisters when
separated can interact with distinct microtubule bundles, but
also that the two bundles frequently originate from opposite
spindle poles. Such merotelically attached kinetochore pairs
would be pulled bidirectionally at anaphase onset and hence
lag behind, leading to aneuploidy. Our study showed that the
degree of sister kinetochore separation correlates strongly with
likelihood of an abnormal merotelic attachment. Because older
eggs feature not only a higher number separated kinetochore
pairs, but also the two kinetochores within each pair are on av-
erage separated by a greater distance, this provided a plausible
explanation for the reduced fidelity of chromosome segregation
in older women.
Figure 3: Sister kidnetochores in human meiosis I become increasingly
more spaced with advancing maternal age
(A, B) Representative images of meiosis I spindles following a cold-treatment
from a young (A; 24 years old) and an older (B; 40 years old) donor. Insets
demonstrate examples of bivalents from the same oocyte as in the overview.
Scale bar represents 5 µm in overview and 1 µm in insets.
(A) Frequency of kinetochore configurations across the different age groups. In
each case, significance analysis was performed by comparing a defined kineto-
chore configuration to its counterpart in the youngest age group. * = p
≤
0.05, **
= p
≤
0.01, *** = p
≤
0.001, **** = p
≤
0.0001.
Mechanism II: human bivalents with separated
kinetochores rotate on meiotic spindles in
older women
Considering that sister kinetochores in human oocytes are
separated to a degree that has not been demonstrated in any
other mammalian species to date, we wondered whether the
unique kinetochore geometry during meiosis I in humans can
further be linked to the unprecedented degree of errors mani-
fested in the female germline.
Importantly, we asked how the meiotic spindle recognizes
the two kinetochores that should form a pair under condi-
tions where sister and non-sister kinetochores of a bivalent
are almost equally spaced. We reasoned that if the increased
spacing of sister kinetochores occasionally resulted in non-sis-
ter kinetochores attaching to a single spindle pole, this would
have detrimental consequences for chromosome segregation
outcomes. Namely, if such bivalents were to subsequently
segregate their chromosomes at anaphase I, two chromatids
of a different parental origin would remain in the egg. As the
fidelity of the second meiotic division relies fully on centromer-
ic cohesins that link kinetochores of sister chromatids only, the
two non-sister kinetochores would remain unpaired throughout
the second meiotic divison, further leading to aneuploidy.
Under circumstances where the pairing of sister kinetochores
became disrupted as described above due to an age-related
deterioration in bivalent architecture, a bivalent could adopt
an unconventional orientation on the meiotic spindle. Namely,
the bivalent would appear rotated by 90 degrees, in contrast
to conventionally aligned in-axis bivalents, in which the two
NIKON YOUNG SCIENTIST AWARD 2018


