Cell News 04/2019
25
development. Strikingly, lymphatic vessels of
Cxcr5
-/-
mice were
filled with intralymphatic CD4
+
clusters (Fig. 3G). Such clusters
were biggest at the presumptive iLN site, but smaller clusters
were also disseminated throughout the lymphatic vascular
network (Fig. 3G). Furthermore, the number of isolated CD4
+
cell
in the skin interstitium was increased (Fig. 3H). Our results thus
show that extravascular LTi cell accumulation is a pre-requisite
for the induction of lymphatic vessel remodeling and LN capsule
formation.
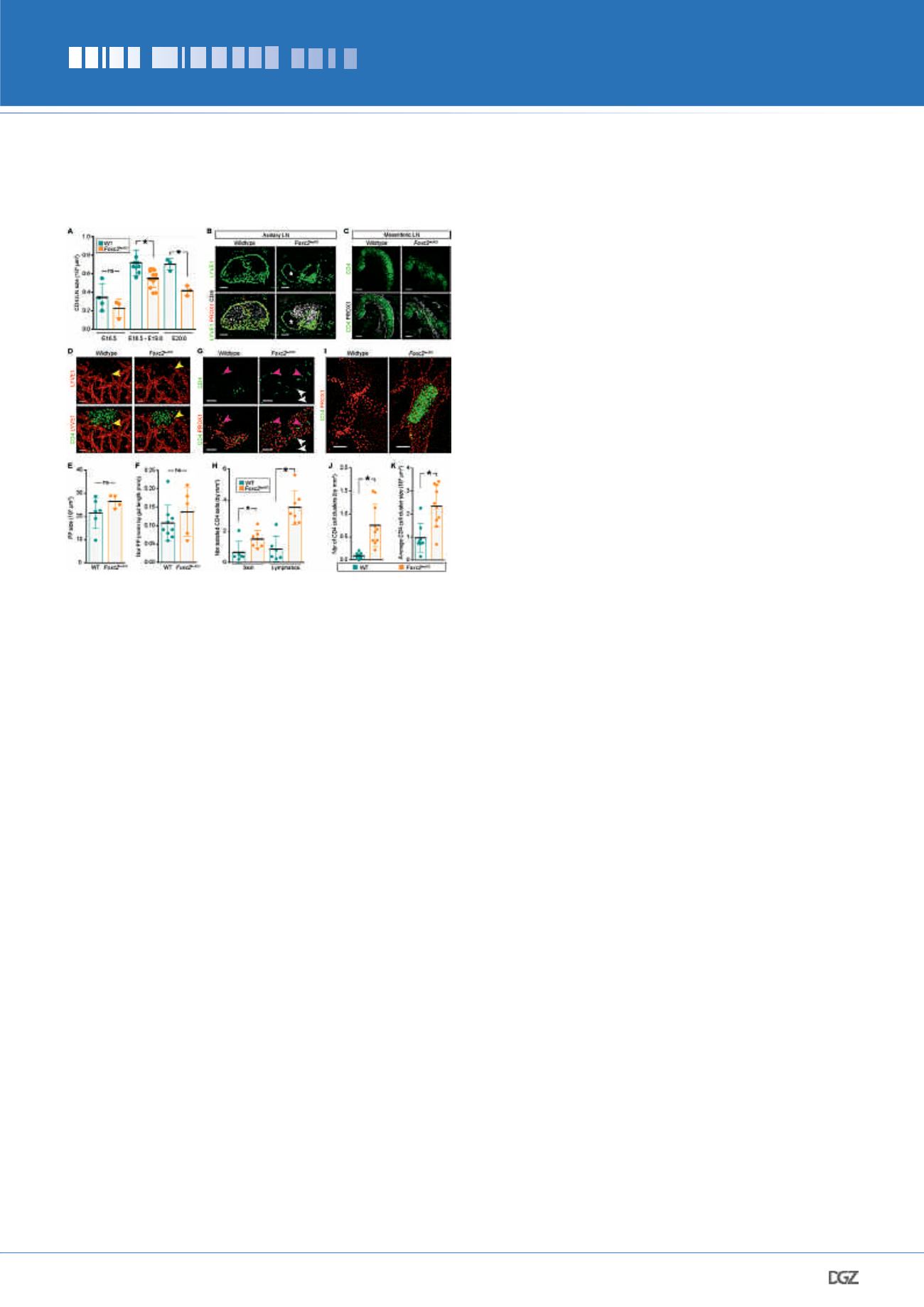
Next, we asked whether disruption of collecting vessel special-
ization in late embryogenesis affects LN development. In
Foxc2
-/-
mice primary lymphatic capillary plexus fails to remodel into
collecting vessels (Norrmén et al., 2009; Petrova et al., 2004).
We analyzed LN development in
Foxc2
f/f
;
Prox1
-CreERT2 (
Fox-
c2
lecKO
) mice with LEC-specific loss of FOXC2. As described pre-
viously (Sabine et al., 2015),
Foxc2
lecKO
embryos had a complete
arrest of collecting vessel development, as determined by the
absence of valves and expression of capillary markers LYVE1 and
CCL21 in all LECs (Fig. 4A). In parallel with the failed collecting
vessel maturation, formation of the LN capsule was severely
disrupted: instead of a continuous lymphatic “cup”, the
Foxc2
lecKO
iLN anlage was surrounded by a mesh-like vasculature, only
partially enclosing the LN (Fig. 4B). In the adult LN, SCS contains
distinct populations of LECs, “ceiling” (cLECs) and “floor” (fLEC)
LECs (Ulvmar et al., 2014). Only cLECs produce the chemokine
receptor CCRL1 while fLECs express LYVE1, ITGA2B and Mad-
CAM1 (Cohen et al., 2010; Cordeiro et al., 2016; Ulvmar et al.,
2014). In embryonic LNs, we identified the same expression
pattern, suggesting that specialization of SCS LECs is established
prenatally (Fig. 4, C-E). Impaired lymphatic vessel maturation,
however, led to a loss of capsule organization. LN LECs in
Fox-
c2
lecKO
mice failed to express SCS LEC markers ITGA2B or CCRL1,
whereas LYVE1 and MadCAM1 were uniformly high (Fig. 4, C-E).
Moreover, mislocalized SMCs encircled the LN lymphatic vessels
(Fig. 4, F and F’). Finally, in adult LNs, the CD169
+
macrophages
inserted into the SCS fLEC layer prevent dissemination of patho-
gens and deliver antigens to adjacent B cells (Carrasco and Ba-
tista, 2007; Junt et al., 2007; Phan et al., 2007). Surprisingly, we
observed CD169
+
macrophages at the bottom of wildtype E18.5
iLN anlagen, close to the lymphatic capsule (Fig. 4G). However,
in
Foxc2
lecKO
mice, the number of macrophages was reduced and
they were dispersed throughout the LN (Fig. 4G). These results
demonstrate that FOXC2 ensures both collecting vessel matura-
tion and pre-natal LN LEC capsule specialization.
From E18.5 onwards the
Foxc2
lecKO
iLNs were significantly smaller
than controls (Fig. 5A). Organization of axillary and mesenteric
LNs was also perturbed, but the formation of non-encapsulated
Peyer’s patches was not affected (Fig. 5, B-F). We next investi-
gated the reason for the reduced
Foxc2
lecKO
LN size. We observed
accumulation of intra- and extra- vascular CD4
+
cells and large
intralymphatic clusters both in the vicinity of LN anlagen and
throughout the dermal lymphatic vessels of
Foxc2
lecKO
mice (Fig
5, G-K). These results demonstrate that collecting vessel matu-
ration is essential for LN capsule formation, SCS specialization
and further LN expansion. We then compared the expression
of chemokines (
Cxcl13, Ccl21
and
Ccl19
), LTi (
Lta
and
Ltb
) and
PRIZE WINNERS 2019
Figure 5. Impaired LN expansion and trafficking of LTi cells in
Foxc2
lecKO
mice.
(A) iLN size in WT and
Foxc2
lecKO
embryos. E16.5,
n
= 4 and 3;
E18.5-E19.0,
n
= 9 and 12; E20.0
n
= 3 and 3 for WT and
Foxc2
lecKO
genotypes, respectively. ns, not significant; *
P
< 0.05. (B) Impaired
axillary LN development in E20.5
Foxc2
lecKO
embryos. Staining for LYVE1
(green), PROX1 (red) and CD4 (white). Asterisk, swollen LN lymphatics
in
Foxc2
lecKO
mice.
n
= 2 per genotype. Scale bar, 100 μm. (C) Impaired
mesenteric LN development in E18.5
Foxc2
lecKO
embryos. Staining for
CD4 (green) and PROX1 (white), 10-μm transverse sections.
n
= 4
per genotype. Scale bar, 200 μm. (D) Normal development of Peyer’s
patches (PPs) in E19.0
Foxc2
lecKO
embryos. Whole-mount staining for
CD4 (green) and LYVE1 (red). Arrowheads, sprouting LECs. WT
n
= 4;
Foxc2
lecKO
n
= 3. Scale bar, 50 μm. (E) Quantification of E18.5 PP size.
WT
n
= 6;
Foxc2
lecKO
n
= 4; ns, not significant. (F) Quantification of
E18.5 PP number. WT
n
= 9;
Foxc2
lecKO
n
= 5; ns, not significant. (G)
Increased isolated CD4+ LTi cells in
Foxc2
lecKO
dermis and lymphatic
vessels. Whole-mount E18.5 skin; CD4 (green) and PROX1 (red). Pink
arrowheads, intralymphatic CD4 cells; white arrowheads, extravascular
CD4 cells. WT
n
= 3;
Foxc2
lecKO
n
= 4. Scale bar, 50 μm. (H) Quantifica-
tion of isolated extra- and intra-lymphatic CD4
+
cells in E18.5-E19.0
WT and
Foxc2
lecKO
embryos. WT
n
= 6 and
Foxc2
lecKO
n
= 7. *
P
< 0.05. (I)
Intralymphatic CD4
+
clusters in skin of
Foxc2
lecKO
embryo. Whole-mount
staining for CD4 (green) and PROX1 (red). E19.0 WT
n
= 3;
Foxc2
lecKO
n
= 4. Scale bar, 50 μm. (J) Quantification of intralymphatic CD4
+
clusters in skin of E18.5-19.0 WT (
n
= 9) and
Foxc2
lecKO
(
n
= 10) mice.
*
P
< 0.05. (K) CD4
+
intralymphatic cluster size in E18.5-E19.0 skin of
WT (
n
= 9) or
Foxc2
lecKO
(
n
= 10) embryos. *
P
< 0.05. All quantifications,
2-tailed unpaired Student’s t test.


