Cell News 2/2014
20
in their function as basal bodies for cilia formation, while their
mitotic spindle poles are devoid of centrioles (Pearson and Wi-
ney, 2009). Thus, it is possible that centrioles were initially only
passive passengers of spindle poles, whereby their association
with the spindle ensured their equal distribution into the two
daughter cells (Friedländer and Wahrman, 1970; Pickett-Heaps,
1971; Debec et al., 2010). This view is supported by the fact
that centrioles are dispensable for mitotic spindle formation
as it has been shown e.g. by laser ablation experiments verteb-
rate cells (Khodjakov et al., 2000; Khodjakov and Rieder, 2001)
or RNAi-based depletion of the essential centriole component
DSas-4 in Drosophila (Basto et al., 2006). However, in these
experiments it became clear that centrioles are required for the
formation of astral microtubules and cilia.
Cavalier-Smith proposed that the precursor of centrosomes
in the prekaryote was a membrane and chromatin-associated
microtubule nucleation center with a dual centromere/centro-
some function (Cavalier-Smith, 2010). It has duplicated during
eukaryotic evolution, with a centrosome staying attached to
the plasma membrane associated with ciliary microtubules and
microtubules building the pellicula and a microtubule nuclea-
tion center attached to endomembranes, which later built up
the nuclear envelope. This may originally have led to an in-
tranuclear microtubule nucleation center whose function was
organization of the intranuclear spindle and an extranuclear
centrosome stabilizing the cell surface through organization
and attachment of pellicular microtubules and a motile cilium/
flagellum, respectively. This split of labor of an intranuclear mi-
crotubule nucleation center and an extranuclear centrosome is
realized for example in discicristata such as Euglena and trypa-
nosomes (Ratcliffe, 1927). In this light the tight association of
a nucleus-associated centrosome with clustered centromeres
during the entire cell cycle as in fission yeast or the amoebozo-
an Dictyostelium would be a primitive attribute. Cavalier-Smith
(Cavalier-Smith, 2010) also suggested that centrin played a key
role in the assembly of the primitive microtubule nucleation
complex at centromeres. Centrins belong to the calmodulin fa-
mily of calcium-binding proteins and are ancient eukaryotic
signature proteins (Hartman and Fedorov, 2002). Their function
can generally be described as connectors between microtubu-
lar structures and membrane-bound structures. Thus, they are
constituents of calcium-sensitive fibers connecting basal bo-
dies to membranes and they play a role in centrosome duplica-
tion as for example in budding yeast, where Cdc31p (the yeast
centrin) is a major constituent of the half bridge, which serves
as the assembly platform for the nascent new SPB upon SPB
duplication. There are several centrin isoforms (four in human
cells) that in most species can be grouped into two subfamilies,
one comprising human centrin-2-like proteins and one com-
prising yeast Cdc31p/centrin-3-like proteins. These subfamilies
obviously arose very early in eukaryotic evolution since their
members are present in both unikonts and bikonts (Bornens
and Azimzadeh, 2007). Thus, loss of one subfamily is likely to
be a secondary effect. Cavalier-Smith suggested that loss of
centrin paralogues in yeasts occurred upon loss of centrioles/
basal bodies with the attached rootlets and pellicle structures
except one paralogue associated with the nuclear centrosome,
where it is required for centrosome attachment to the nucleus
and centrosome duplication (Cavalier-Smith, 2010). Yet, one
cannot generalize a role of the centrin-3 like isoforms for cen-
trosome duplication and nuclear functions, since e.g. in flies
and nematodes centrin-3 is missing and centrin-2 is required
for this job (Bornens and Azimzadeh, 2007). Furthermore, it is
centrin-2 which serves a function in nucleotide excision re-
pair after DNA damage as part of the xeroderma pigmentosum
group C complex (XPC complex) within the nucleus (Araki et al.,
2001; Dantas et al., 2012). The two existing centrin paralogues
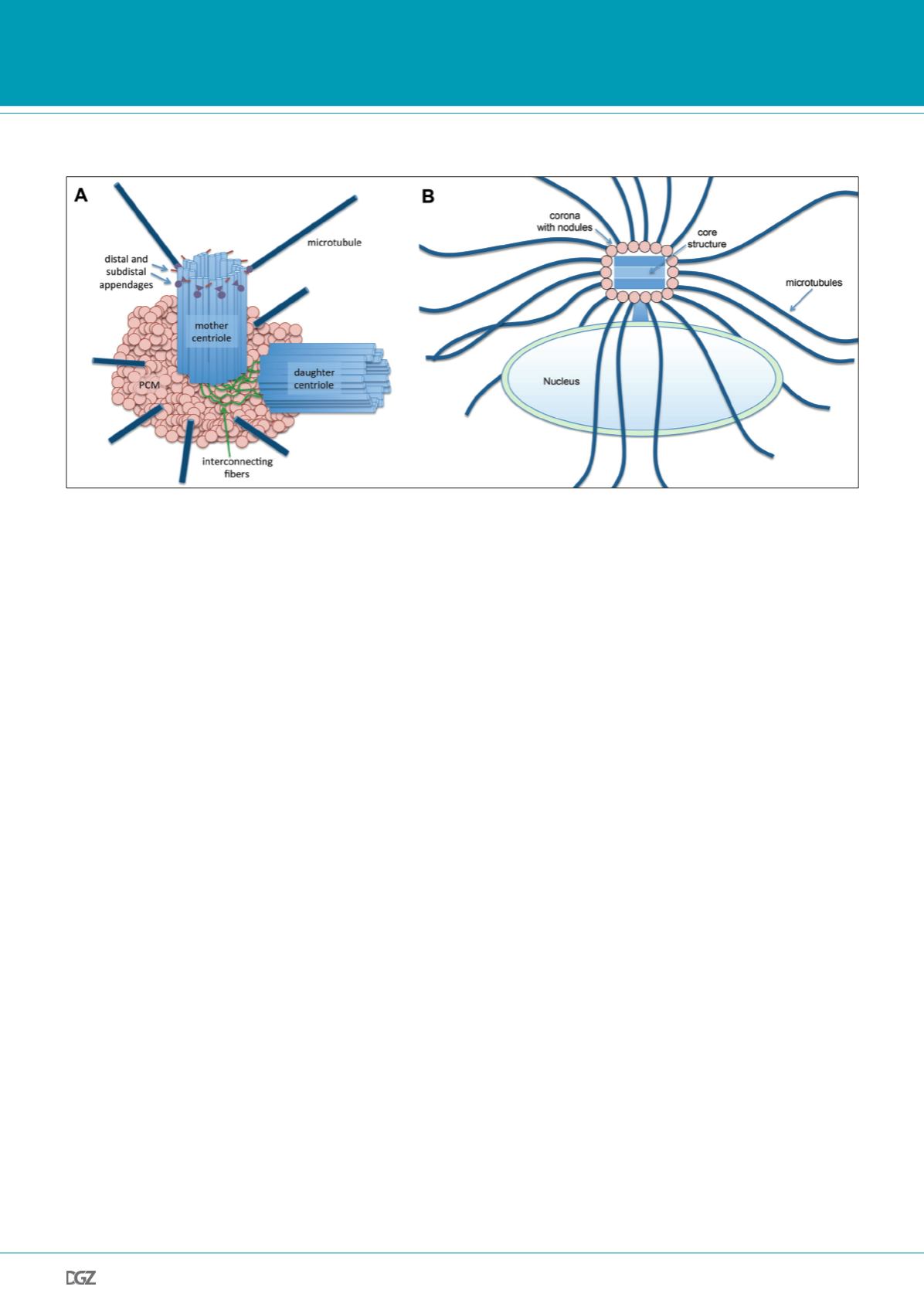
Figure 2.
Different centrosome types of animals and Dictyostelium. (A) Centriole-containing animal centrosome. (B) Acentriolar Dictyostelium centrosome. See text
for further descriptions.
Research news


