Cell News 2/2014
12
Research news
known reduction of microtubules in AD and might contribute
to neurodegeneration in other Tau-dependent pathological
states. The mechanistic description could lead to the develop-
ment of new therapeutic strategies. For example, we showed
that downregulation of spastin prevents loss of microtubules
and missorting of Tau, two key events in Alzheimer pathology.
About the author
Hans Zempel studied Biochemistry at the Berlin Free University
and obtained his MSc in the group of Ulrich Pison at the Chari-
té, Berlin. He then moved to Japan to study the clinical part of
medical school in Tokyo Medical and Dental University. For his
PhD he then joined the laboratory of Eva-Maria and Eckhard
Mandelkow at the Max-Planck-Unit for Structural Molecular
Biology at DESY, Hamburg, and later at the German Center for
Neurodegenerative Disease at CAESAR, Bonn, where he worked
on the analysis of cell models of Alzheimer Disease and Fronto-
temporal Dementia.
Acknowledgements
Work presented here is the result of many members of the
Mandelkow Lab. Of particular importance is Julia Luedtke, who
helped in many aspects of this work. XiaoYu Li and Yatender
Kumar developed and drove the discovery and investigation of
the Tau Diffusion Barrier. Thomas Timm developed assay systems
and imaging techniques to investigate MARK and to screen for
MARK inhibitors. Jacek Biernat with help from Sabrina Hübsch-
mann was instrumental for cloning of different Tau and MARK
constructs, and adenovirus production. Funding was from MPG,
DZNE, KNDD, MEMOSAD (FP7), Metlife Foundation, Tau Con-
sortium.
References
Bloom, G.S. 2014. Amyloid-beta and Tau: The Trigger and Bullet in Alzheimer Disease Patho-
genesis. JAMA neurology. 71:505-508.
Haass, C., and D.J. Selkoe. 2007. Soluble protein oligomers in neurodegeneration: lessons
from the Alzheimer's amyloid beta-peptide. Nature reviews. Molecular cell biology. 8:101-
112.
Ittner, L.M., and J. Gotz. 2010. Amyloid-beta and tau - a toxic pas de deux in Alzheimer's
disease. Nature reviews.
Jean, D.C., and P.W. Baas. 2013. It cuts two ways: microtubule loss during Alzheimer disease.
The EMBO journal. 32:2900-2902.
Kanai, Y., and N. Hirokawa. 1995. Sorting mechanisms of tau and MAP2 in neurons: sup-
pressed axonal transit of MAP2 and locally regulated microtubule binding. Neuron. 14:421-
432.
Karran, E., M. Mercken, and B. De Strooper. 2011. The amyloid cascade hypothesis for
Alzheimer's disease: an appraisal for the development of therapeutics. Nature reviews. Drug
discovery. 10:698-712.
Lacroix, B., J. van Dijk, N.D. Gold, J. Guizetti, G. Aldrian-Herrada, K. Rogowski, D.W. Gerlich,
and C. Janke. 2010. Tubulin polyglutamylation stimulates spastin-mediated microtubule
severing. The Journal of cell biology. 189:945-954.
Li, X., Y. Kumar, H. Zempel, E.M. Mandelkow, J. Biernat, and E. Mandelkow. 2011. Novel
diffusion barrier for axonal retention of Tau in neurons and its failure in neurodegeneration.
The EMBO journal. 30:4825-4837.
Mandelkow, E.M., and E. Mandelkow. 2012. Biochemistry and cell biology of tau protein
in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2:a006247.
Morris, M., S. Maeda, K. Vossel, and L. Mucke. 2011. The many faces of tau. Neuron. 70:410-
426.
Thies, E., and E.M. Mandelkow. 2007. Missorting of tau in neurons causes degeneration of
synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci. 27:2896-2907.
Timm, T., J.P. von Kries, X. Li, H. Zempel, E. Mandelkow, and E.M. Mandelkow. 2011. Mi-
crotubule affinity regulating kinase activity in living neurons was examined by a gene-
tically encoded fluorescence resonance energy transfer/fluorescence lifetime imaging-
based biosensor: inhibitors with therapeutic potential. The Journal of biological chemistry.
286:41711-41722.
Zempel, H., and E.M. Mandelkow. 2012. Linking amyloid-beta and tau: amyloid-beta indu-
ced synaptic dysfunction via local wreckage of the neuronal cytoskeleton. Neuro-degene-
rative diseases. 10:64-72.
Zempel, H., E. Thies, E. Mandelkow, and E.M. Mandelkow. 2010. Abeta oligomers cause loca-
lized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation,
and destruction of microtubules and spines. J Neurosci. 30:11938-11950.
Zempel, H., J. Luedtke, Y. Kumar, J. Biernat, H. Dawson, E. Mandelkow, and E.M. Mandelkow.
2013. Amyloid-beta oligomers induce synaptic damage via Tau-dependent microtubule se-
vering by TTLL6 and spastin. The EMBO journal. 32:2920-2937.
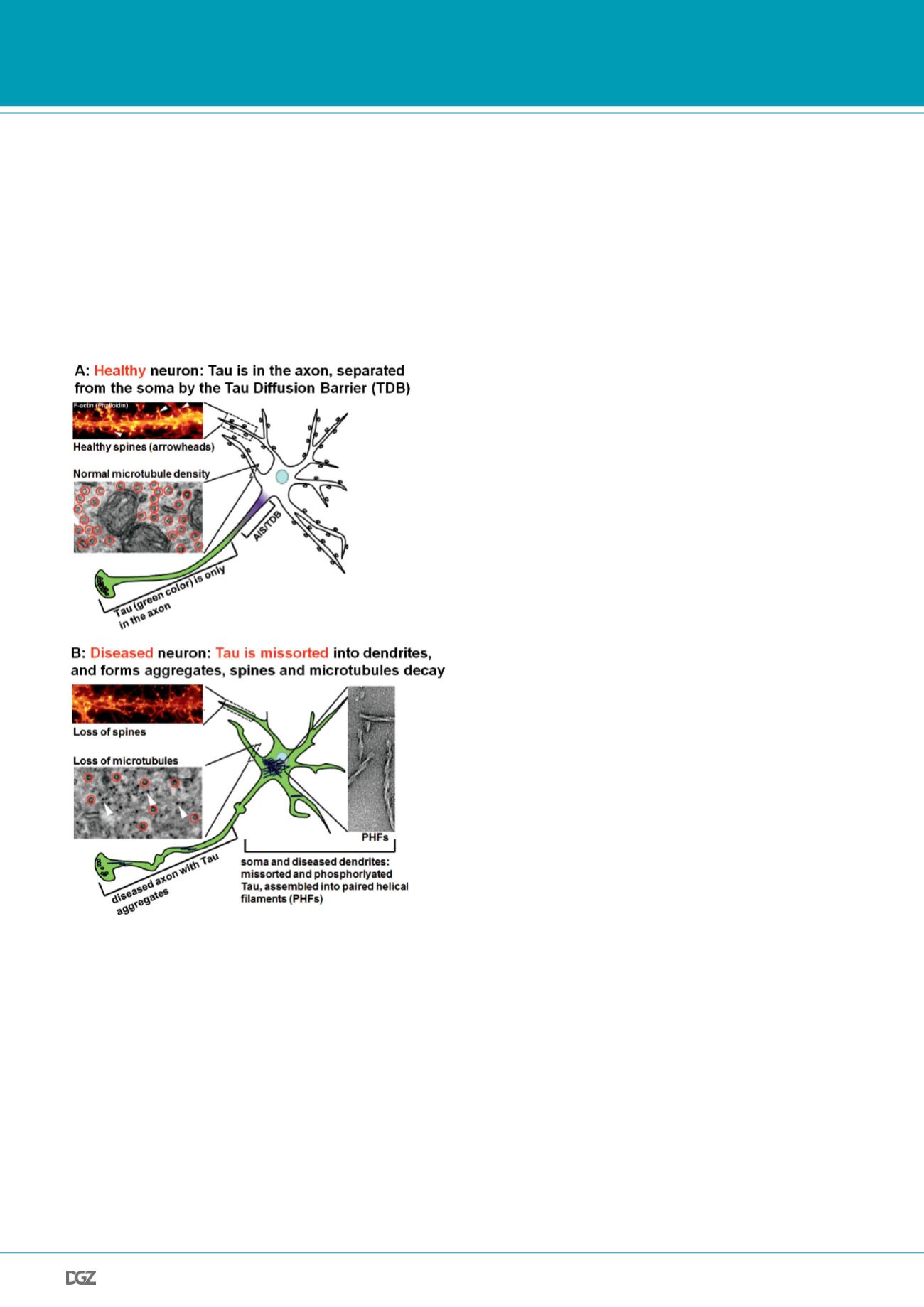
Figure 3. In Alzheimer Disease, Tau is missorted into the soma and the
dendrites, causing loss of spines and microtubules.
A: In mature healthy neurons, the Tau diffusion barrier (TDB, depicted in
magenta) within the axon initial segment (AIS) is established, Tau (green)
is sorted into the axon. Dendrites display dense spines (upper panel, spine
staining with phalloidin) and dense microtubules (lower panel: highlighted
by red circles in cross sectioned dendrite imaged by electron microscopy
(TEM)).
B: In diseased or stressed neurons (e.g. exposed to elevated A
β
as shown
here, Tau mutations or traumatic injury), Tau (green) is missorted into the
soma and the dendrites, axons become distorted and develop varicosities.
Dendrites retract, lose their spines (upper panel), and microtubule density
(lower panel, red circles) is reduced. Dendrites are invaded by neurofi-
laments (NF, indicated by white arrowheads). Missorted Tau in diseased
neurons assembles into paired helical filaments (PHFs, panel to the right:
TEM of aggregated recombinant human Tau).
Images are modified from (Mandelkow and Mandelkow, 2012; Zempel et
al., 2013; Zempel et al., 2010).


