Cell News 04/2019
10
PRIZE WINNERS 2019
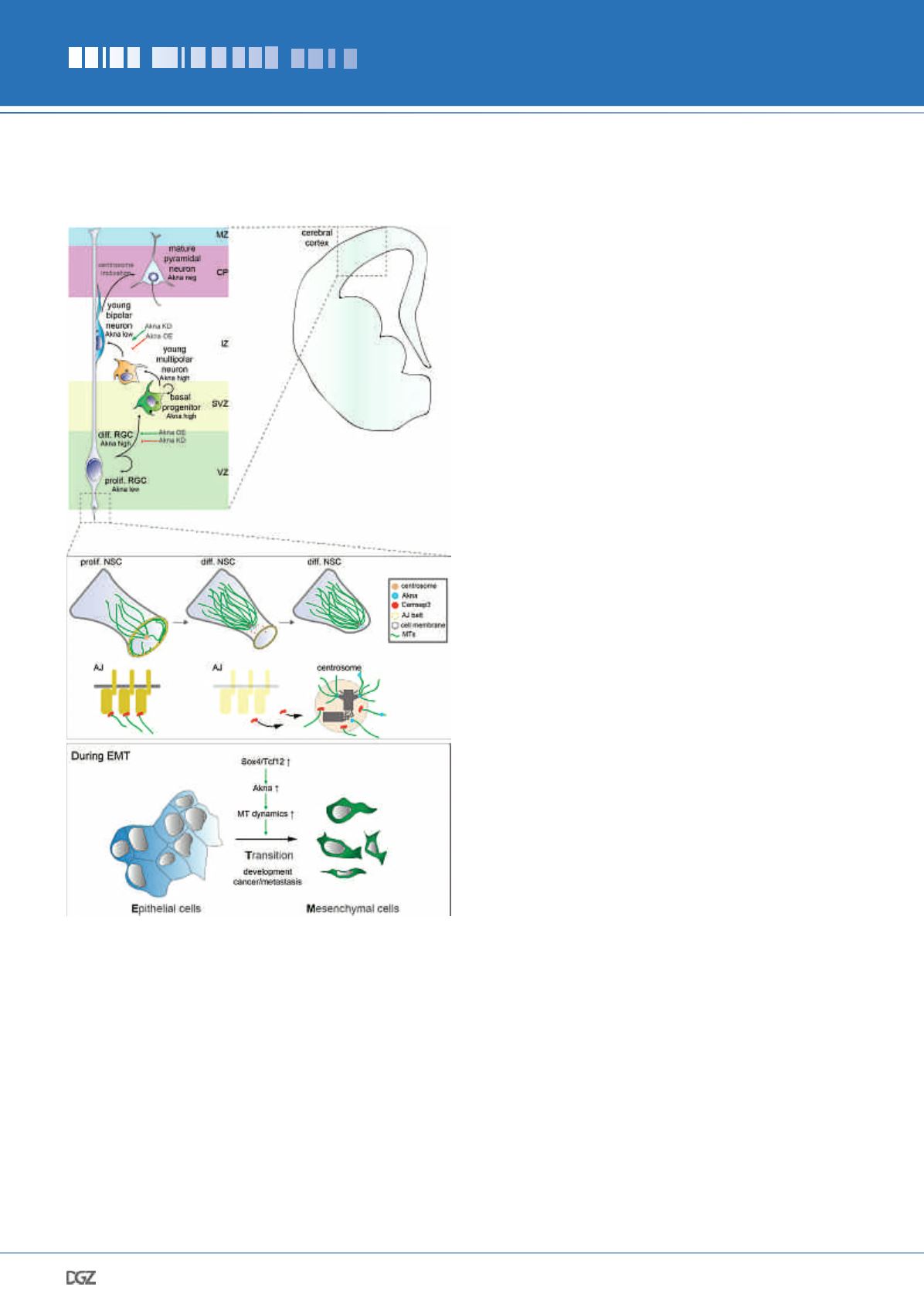
Down-regulation of Akna impairs delamination of differen-
tiating NSCs, as they largely remain undifferentiated in the
VZ (consequently there is less generation of BPs). In contrast,
upregulation of Akna promotes NSC delamination towards the
SVZ as well as their differentiation into BPs (hence less prolif-
erating NSCs remain at the VZ) (Figure 3b and summarized in
Figure 4). Importantly, the delamination happens by retraction
of the apical process
during interphase
(when Akna is present at
centrosomes), not during mitosis (when Akna is not observed at
mitotic spindles). Importantly (see discussion above), the centro-
somal localization of Akna is key in the delamination phenotype,
since the overexpression of a construct lacking the c-terminal,
centrosome targeting, region has no effect.
These results prompted us to investigate the cellular mech-
anisms driving Akna-mediated delamination. Given Akna’s
enrichment at SDAs - the place at centrosomes where MTs are
organized - we checked which features of MTs are affected
upon manipulation of Akna. Using a combination of
in vivo
and
in vitro
bio-imaging assays, we uncovered that Akna
promotes
centrosomal MT growth, nucleation and organization; hence,
MTs are more dynamic when Akna is highly expressed. In con-
trast, lowering Akna levels reduces the cell’s ability to nucleate
centrosomal MTs and reduce the polymerization speed; i.e. MTs
are less dynamic.
Akna functions through the recruitment of proteins that polym-
erize and nucleate MT like the gamma-Tubulin Ring Complex
and Ckap5 (the mammalian homolog of XMAP215), and MT
organizing proteins such as Odf2, Mapre1, and the Dynein/Dy-
nactin complex, among others. Importantly, Akna can also bind
MTs directly, promoting their growth and reducing shrinking.
Moreover, Akna can very efficiently recruit the MT minus-end
binding proteins Camsap3, which in epithelial cells (such as
NSCs!), organizes MTs at adherens junctions (AJs). In the ab-
sence of Camsap3 AJs fall apart, cells lose attachment to each
other and concomitantly delaminate (Dong et al. 2017; Meng et
al., 2008). We thus speculated that an increase in centrosomal
MT dynamics mediated by Akna could lead to the re-localiza-
tion of Camsap3 towards the centrosome, thereby decreasing
AJs stability, inducing the constriction of NSC apical end-feet
(Kasioulis et al., 2017) and ultimately the retraction of the apical
process, leading to delamination. Indeed, endfeet of NSCs with
Akna at the centrosome were smaller than endfeet of NSCs
without/low level of Akna at the centrosome, and they become
even smaller upon Akna OE. In addition, Akna overexpression
reduces Cadherin levels at the apical surface. Treatment of NSCs
in vivo with the microtubule stabilizing agent Taxol rescues the
phenotype of Akna overexpression, demonstrating that enhanced
MT dynamics are essential for delamination (summarized in
Figure 4).
Since this process resembles the epithelial-to-mesenchymal
transition (EMT) (Ito et al., 2013; Signh et al., 2016; Zander et
al., 2014), we asked whether Akna is more generally relevant for
EMT by monitoring normal murine mammary gland (NMuMG)
epithelial cells undergoing EMT induced by Transforming Growth
Factor Beta 1 (TGF
β
1). Here, we first observed that centrosomal
Akna protein levels are upregulated early in EMT compared to
untreated cells. Notably, NMuMG cells have largely non-centro-
somal MT polymerization, and increasing Akna levels strongly
re-organized MT growth to the centrosome, in accordance to the
abovementioned observation in neural cells. Moreover, knock-
ing down Akna counteracted the EMT-induced disassembly of
cell junctions, along with retaining Camsap3 at AJs. Conversely,
Akna was sufficient to reduce Camsap3 at the junctional inter-
face closest to AKNA+ foci upon overexpression, but not Cam-
sap3 at more-distant junctions. Together, this suggest that Akna
mediates the remodeling of junctional complexes by recruiting
Camsap3 from junctional microtubules to the centrosome,
Figure 4:
Summary of the role of Akna in the developing cerebral
cortex and in epithelial cells during EMT.


